Addition Reaction of Alkenes with Hydrogen
Alkenes contain at least one carbon-carbon double bond. It is the presence of this double bond that makes alkenes more reactive than alkanes. As was demonstrated by the test for alkenes using bromine water, all alkenes can be characterised by their addition reactions. Addition reactions involving alkenes are reactions in which the carbon–carbon double bond is converted to a single bond and atoms or groups are added to each of the two carbon atoms.
Alkenes undergo an addition reaction with hydrogen in the presence of a catalyst to form a saturated compound.
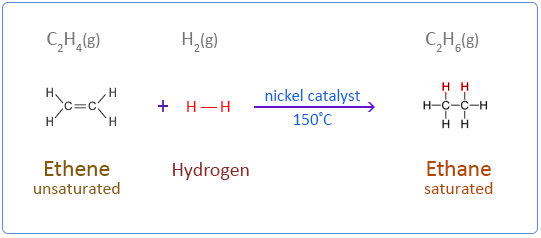
Ethene does not react with hydrogen under normal conditions. But in the presence of a catalyst such as nickel the reaction takes place at 150°C. Palladium is a more effective catalyst than nickel but nickel is the preferred choice in industry as it is less expensive. This type of addition reaction is called catalytic hydrogenation.
Catalytic hydrogenation using a nickel powder catalyst is used in the food industry to convert edible plant and vegetable oils into edible fats or margarine. Vegetable or plants oils such as sunflower seed oil or palm oil consist of long chain hydrocarbon molecules which contain double bonds. Although these are useful for cooking, these oils do not hold the same commercial value as expensive animal fats such as butter. Through the addition reaction with hydrogen in the presence of a nickel catalyst the double bonds in these oils are broken and the molecules become saturated. This increases the melting point of the oil so that it becomes a solid i.e. a fat at room temperature. The degree of the softness of the margarine can be controlled by regulating the amount of hydrogenation or in other words the number of double bonds in the hydrocarbon molecule.
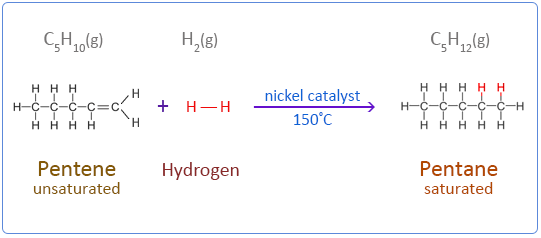
Below are the chemical equations for the first four members of the alkenes homologous series addition reactions with hydrogen.
Ethene
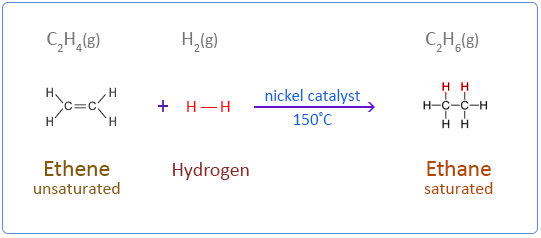
Propene
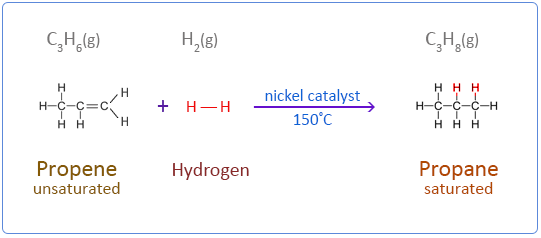
Butene
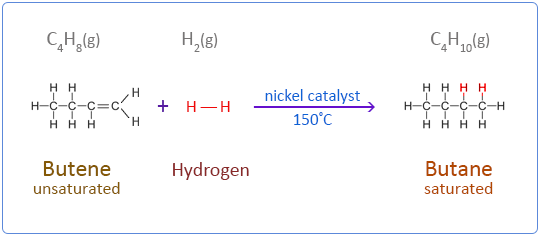
Pentene