Carboxylic Acids
Carboxylic acids contain the carboxyl group:
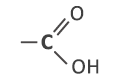
This is a combination of the carbonyl group and hydroxyl group:
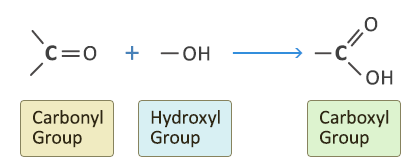
Carboxylic Acid Homologous Series
Carboxylic acids with one carboxyl group are called monocarboxylic acids. They are named by replacing the final “e” of the corresponding alkane by “oic acid”. A hydrocarbon homologous series is a series of hydrocarbons which:
- Have the same general formula
- Differ by CH2 in molecular formulae from neighbouring compounds
- Show a gradual variation in physical properties i.e. boiling and melting point
- Have similar chemical properties.
The similarity in chemical properties of a homologous series is because the compounds contain the same functional group. In the case of the carboxylic acids it is the carboxyl group –COOH.
The table below shows the first four carboxylic acids in the monocarboxylic acid homologous series.
| Name | Number of Carbon atoms | Molecular Formula | Structural Formula |
|---|---|---|---|
| Methanoic Acid formerly called formic acid, from the Latin formica, an ant. Ants inject methanoic acid when they bite their victims, hence the former name. |
1 | HCOOH | 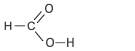 |
| Ethanoic Acid formerly called acetic acid, from the Latin acetum, vinegar. Ethanoic acid is present in vinegar. |
2 | CH3COOH | 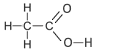 |
| Propanoic Acid formerly called propionic acid. |
3 | C2H5COOH | 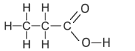 |
| Butanoic Acid | 4 | C3H7COOH | 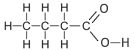 |
