Chemical Properties of Alkali Metals
The alkali metals are the most reactive group of metals in the Periodic Table. They contain one weakly held electron in their outer shell which is readily transferred in chemical reactions.
Reactions of alkali metals with water
All the Group 1 elements react vigorously with cold water. They react with water to form hydrogen gas and an alkaline solution of the metal hydroxide. All the Group 1 elements readily give up their weakly held outermost electron resulting in a positive metal ion with a full outer shell i.e. the stable electronic arrangement of a noble gas. In a reaction, an atom of a Group 1 element loses one electron and forms an ion with a single positive charge. The process of losing an electron is termed as oxidation.
If we consider the example of sodium reacting with water:
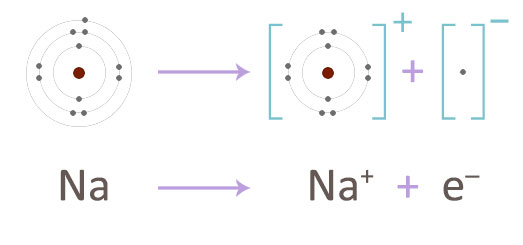
The sodium atom readily gives up its electron to form a sodium ion.
The water molecule is then reduced by the sodium ion as follows:
H2O (l) + e– —→ ½H2(g) + OH–
The Na+ ion combines with the OH– ion through ionic bonding to form sodium hydroxide (NaOH).
The reactivity of the alkali metals increases going down Group 1.
The animation below shows the reactions of lithium (Li), sodium (Na) and potassium (K) with water:
Reactions of alkali metals with chlorine
All the Group 1 elements react vigorously with chlorine. They react with chlorine to form white crystalline salts.
Lithium
If a piece of hot lithium is lowered into a jar of chlorine, a vigorous reaction takes place forming white powder that settles on the sides of the jar. This is the salt lithium chloride (LiCl). The reaction can be written in the form of an equation:
lithium + chlorine → lithium chloride
2Li(s) + Cl2(g) → 2LiCl(s)
Sodium
The reaction of sodium with chlorine is more vigorous than lithium. If a piece of hot sodium is lowered into a jar of chlorine, the sodium burns with a bright yellow flame forming clouds of white powder that settle on the sides of the jar. This is the salt sodium chloride (NaCl).
The reaction of sodium with chlorine can be written as:
sodium + chlorine → sodium chloride
2Na(s) + Cl2(g) → 2NaCl(s)
Potassium
Potassium reacts more violently with chlorine than sodium does, showing how reactivity increases down the group. The salt potassium chloride (KCl) is produced by this reaction. The reaction can be written as:
potassium + chlorine → potassium chloride
2K(s) + Cl2(g) → 2KCl(s)
