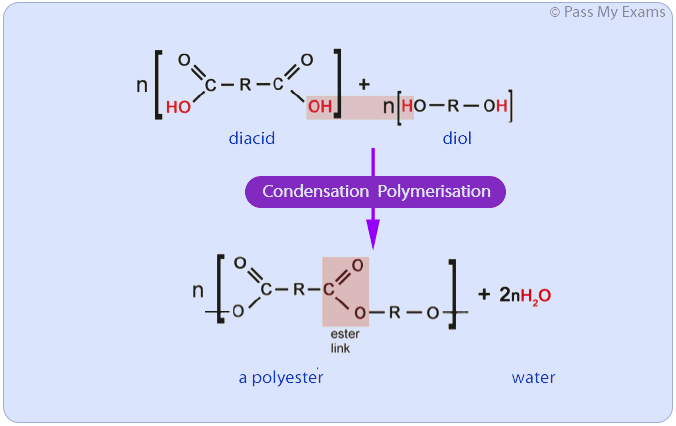
Condensation Polymerisation
Condensation polymerisation involves a reaction between monomers with two different functional groups. When these types of monomers react they join together, usually losing a small molecule such as water, and so the reactions are called condensation reactions.
Most natural polymers are condensation polymers, and many synthetic polymers are made by condensation polymerisation as substitutes to natural fibres such as wool and silk.
Example: Polyesters
Polyesters are examples of a polymers produced from condensation polymerisation. Polyesters are formed by the reactions between a carboxylic acid containing two carboxyl functional groups and an alcohol containing two hydroxyl groups.
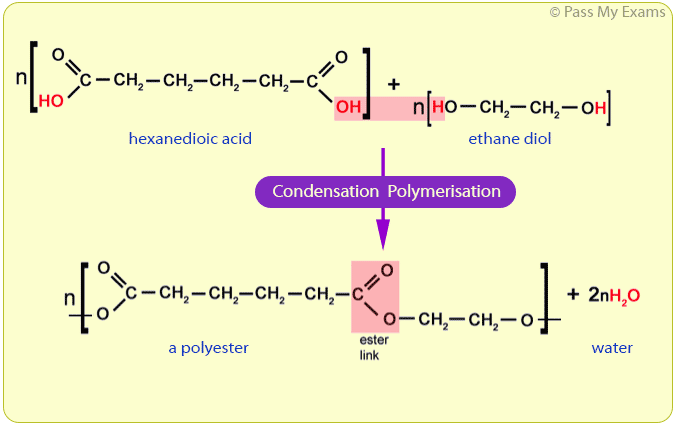
For example, ethane diol and hexanedioic acid undergo condensation polymerisation to form a polyester.
Ethane diol:
HO — CH2 — CH2 — OH
Ethane diol is an alcohol with two hydroxyl functional groups, hence the name diol (di meaning two, and ol for the hydroxyl groups).
Hexanedioic acid:
HOOC — CH2 — CH2 — CH2 — CH2 — COOH
Hexanedioic acid is a carboxylic acid with two carboxyl functional groups, hence the name dioic (di meaning two, and oic for the carboxyl groups).
Polyesters can be formed by a condensation polymerisation reaction between a carboxylic acid monomer and an alcohol monomer if both monomers have two functional groups. A carboxylic acid with two carboxyl groups is referred to as a diacid and an alcohol with two hydroxyl groups is referred to as a diol.