Explaining trends in reactivity
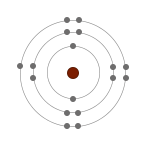
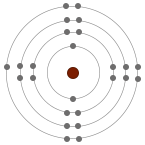
The reactivity of Group 7 elements decreases down the group. Non-metal atoms gain electrons when they react with metals. When a halogen atom reacts, it gains one electron into their highest occupied energy level (outer shell) to form a singly negative charged ion.
As we go down the group, an additional electron shell is added thereby increasing the atomic radii of the atom. The electrons in the outer shell move further away from the nucleus as we go down the group and the attraction force between the electrons and the nucleus become weaker and weaker. This weaker attraction in the larger atoms makes it harder to gain electron. Therefore the ability of the atom to attract electron to fill the outermost shell reduces, which means the reactiveness of the atom reduces.
| Chlorine (Cl) | Bromine (Br) | Iodine (I) |
|---|---|---|
 |
 |
 |
| 2.8.7 | 2.8.8.7 | 2.8.8.8.7 |
| Chlorine is more reactive than iodine although they both need to gain only one electron to have full outer shells. It is because the outer electron of iodine atom is furthest from the positive attractions of the nucleus compared to the outer electron of chlorine. Greater distance between nucleus and outer shell means less attraction so it is harder to gain an extra electron. Therefore, it is easier for chlorine to gain an electron and form a halide. | ||
