Development of the Periodic Table
Some 200 years ago scientists were discovering lots of new elements. The challenge they faced at the time was the difficulty in finding any links between the different elements. It must be remembered this was well before the atomic structure of elements were understood. However, the Relative Atomic Masses of elements had only recently been discovered and through experimentation scientist could identify similar behaviors and patterns. It was this attempt to try to link and classify elements that led to the development of the periodic table.
The work of many scientists contributed to the present form of the Periodic Table. The timeline below shows the significant stages in the history of the development of the Periodic Table.
1829
Döbereiner’s triads
One of the earliest attempts to classify the elements was put together by a German chemist Johann Wolfgang Döbereiner. In 1829, he noticed that particular groups of three elements with similar chemical properties had atomic masses, such that the atomic mass of the middle member was approximately the mean of the other two. He named the group of three similar elements triads. Döbereiner's work encouraged other scientists to search for correlations between the chemical properties of the elements and their atomic weights.
Example of Döbereiner triads:
| Lithium 7 |
Sodium 23 |
Potassium 39 |
| Calcium 40 |
Strontium 87.5 |
Barium 137.5 |
| Sulphur 32 |
Selenium 79 |
Tellurium 127.5 |
| Chlorine 35.5 |
Bromine 80 |
Iodine 127 |
1865
Newlands' octaves
In 1864, an English scientist named John Newlands noticed that if the known elements were written down in ascending order of atomic mass, similar properties were repeated in every eight element, similar to notes on a musical scale. He called it his Law of Octaves.
Newlands arranged the elements in a table containing seven columns so every eighth element having similar properties appeared in the same vertical column called groups. By ordering the elements strictly according to atomic mass, he was forced to put some elements into groups having dissimilar properties. As a result, his table was not accepted by the scientific community.
Newlands’ octaves:
| H | Li | Be | B | C | N | O |
| F | Na | Mg | Al | Si | P | S |
| Cl | K | Ca | Cr | Ti | Mn | Fe |
| Co and Ni | Cu | Zn | Y | In | As | Se |
| Br | Rb | Sr | Ce and La | Zr | Di and Mo | Ro and Ru |
Newlands system worked quite well for the light elements but broke down for the heavier elements.
- Newland made the incorrect assumption that all elements had been discovered. The discovery of one more element could throw out the whole idea of octaves. Dr. Gladstone, a peer reviewing Newlands idea, raised the point that four new elements (thallium, indium, caesium and rubidium) had been discovered in the few years previous to Newlands suggestion. So, there was the potential for further discoveries to follow.
- In order to make the repeating octaves system work in certain cases Newland had to place two elements in one space.
- Newlands’ classification system grouped heavier elements with dissimilar properties together.
For these reasons Newlands’ ideas were rejected.
1869
Mendeléev's periodic table
In 1869, a Russian chemist named Dmitri Mendeléev published a periodic table. He arranged the elements known at the time in order of increasing relative atomic mass and showed that elements of similar properties reoccurred at regular intervals. The table he produced had elements with similar properties fall into the same vertical column. The vertical columns of similar elements are called groups and the horizontal rows of elements are called periods.
Mendeléev's periodic table:
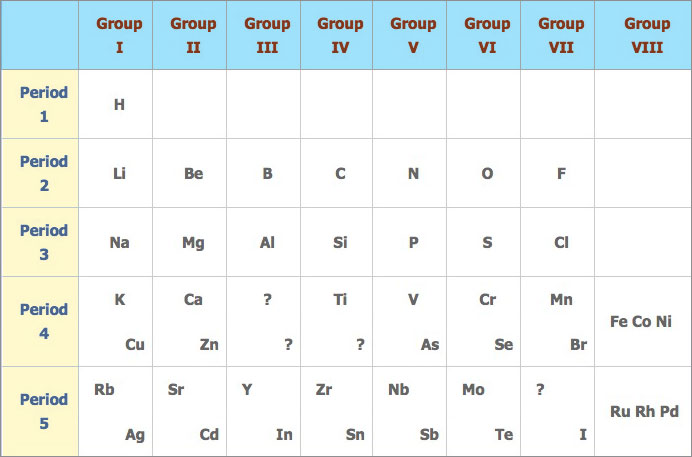
Mendeléev’s periodic table was more successful than the one proposed by Newlands because:
- Mendeléev was more confident on his proposal and was prepared to take risks. Where the pattern didn’t work he left gaps in the table so that similar elements would fall in the same vertical column.
- He claimed that in due course, these gaps will be filled by elements that had not yet been discovered.
- Within his lifetime some of these elements were discovered the properties of which coincided very closely to the predictions made by c.
- Mendeléev also proposed that periods 4, 5, 6 and 7 should contain more than seven elements. To make these longer periods fit to his pattern, he divided them into halves and placed the first half of the elements in the top left hand corner and the second in the bottom right hand corner.
Mendeléev only had the luxury of atomic mass for the basis of his periodic table. He reversed the order of tellurium (Te = 127.6) and iodine (I = 126.9) arguing that iodine must occupy the same group as chlorine and bromine. Since the discovery of electronic structures and atomic numbers his prediction was proven correct as the atomic number of tellurium is 52 and that of iodine 53.
Present
The Periodic Table
The Periodic Table
Mendeléev recognized that in order for the elements to be grouped in patterns he sometimes needed to change the order of the atomic masses. It was not until work carried by Moseley in 1913 that the atomic number of the elements were identified. This discovery resolved the anomalies encountered in Mendeléev’s periodic table and the modern periodic table was established. Off course the important work and contributions of other scientist into the understanding the atomic structure such as J. J Thomson, Geiger and Marsden, Rutherford and Chadwick cannot be ignored.
The modern periodic table has all the elements arranged in strict order of their atomic numbers and therefore can be seen as an arrangement of the elements in terms of their electronic structures.
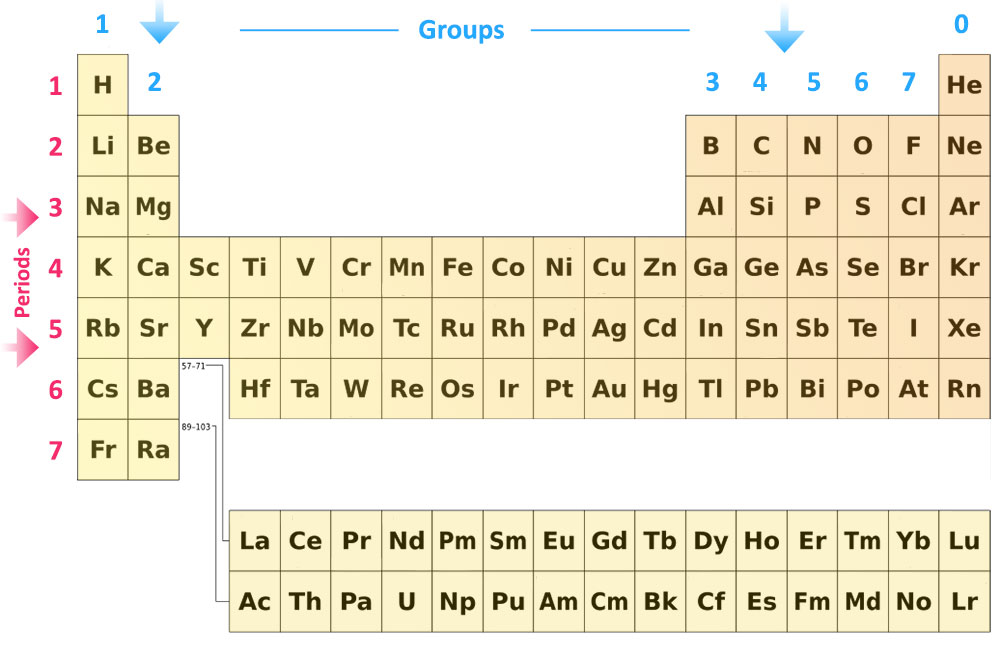
All the elements are placed in order of increasing atomic number in horizontal rows called periods. Arranging the elements in order of their proton numbers gives repeating patterns in the properties of elements. Elements with similar properties are placed in the same vertical column, called groups.
Groups
The vertical columns of the periodic table are called groups. There are eight groups in total. The groups are numbered from 1 to 7 going from left to right, and the eighth group on the right is Group 0.
Elements in the same group have the same number of electrons in their highest energy level (outer shell) and this gives them similar chemical properties.
The elements in Group 1 have only one electron in their outer energy level or shell. Similarly, atoms with two outer shell electrons are placed in Group 2.
Some of the groups have their specific names:
Group 1: The alkali metals.
Group 2: The alkaline earth metals.
Group 7: The halogens.
Group 0: The noble gases.
Periods
The horizontal rows are called periods. The Periods represent the energy shell these atoms outer electrons are located within. It means that all Period 2 elements have their outer electrons in the second energy level or shell; Period 3 elements all have their outer electrons in the third energy level/shell, and so on.
Metals and Non-metals
The metals are placed on the left and the non-metals are placed on the right. Groups 1 and 2 are all metals and groups 7 and 0 only contain non-metals.
The elements in the middle groups begin with non-metals at the top and end with metals at the bottom. Group 4 can be taken as an example to explain this.
| C | Carbon | Non-metal |
| Si | Silicon | Referred to as semi-metals or metalloids due to being on the borderline between metals and non-metals. Silicon is shiny like a metal, but brittle like a non-metal. |
| Ge | Germanium | |
| Sn | Tin | Metal – the oxide of tin shows both metal and non-metal behaviours. It behaves like an acid and base and is called an amphoteric oxide. |
| Pb | Lead | metal |
The metals and non-metals in the periodic table can be divided by drawing an imaginary line like a staircase from boron to astatine. The elements below the stairs are metals and those above are non-metals.
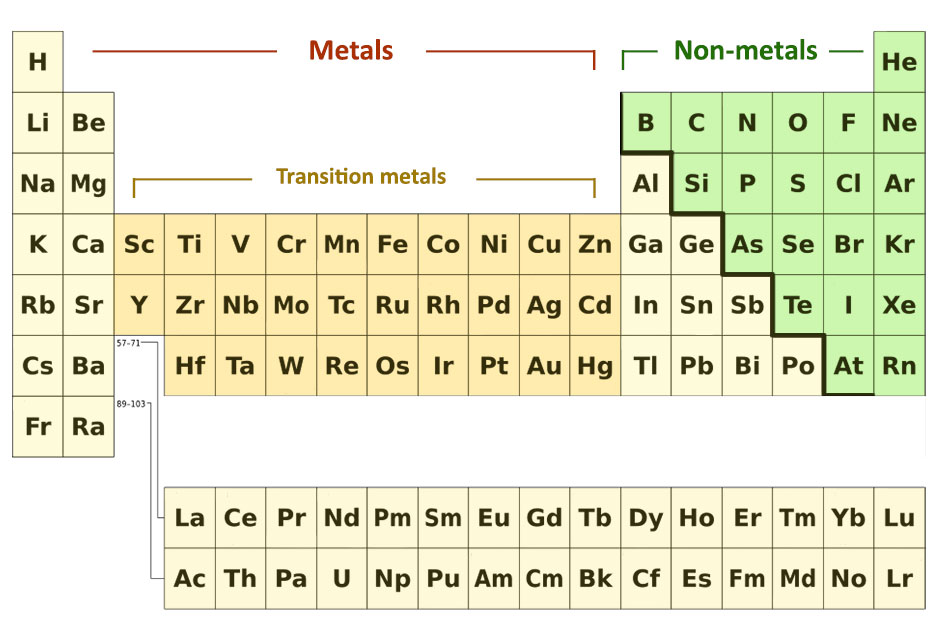
The block of elements in between Group 2 and Group 3 contains the transition metals.
