Detecting radioactivity – the Geiger Muller Tube
Alpha, Gamma and Beta radiations are invisible to humans and exposure to these radiations can be hazardous to the health of living organisms. It is therefore extremely important that suitably designed detectors are available in order to gain information on the type and amount of radiation present.
The Geiger-Muller tube or Geiger counter
Alpha, Beta and Gamma radiations are all ionising radiations. This means that all three forms of radiations have enough energy to pull electrons from atoms turning them into ions. The Geiger-Muller tube makes use of this fact.
The animation below explains how a Geiger-Muller tube works.
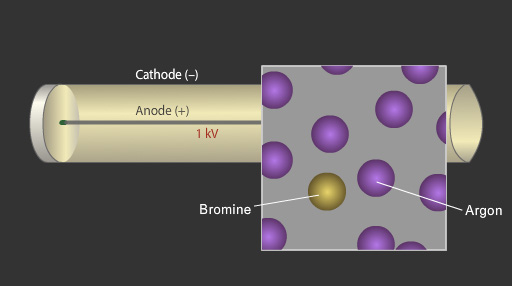
A Geiger-Muller tube consists of a sealed metallic tube filled with argon or another noble gas mixed with a small amount of alcohol vapour or bromine gas. The argon gas is called the detecting gas whereas the bromine gas or alcohol vapours are referred to as the quenching gas. The gas mixture inside the tube is at a pressure below atmospheric pressure. A thin metal wire runs through the centre of the tube. An electric potential of up to 1 kilovolt is maintained between the metal wire (the anode) and the cylinder (the cathode). In the absence of any radiation no current flows between the wire and the cylinder.
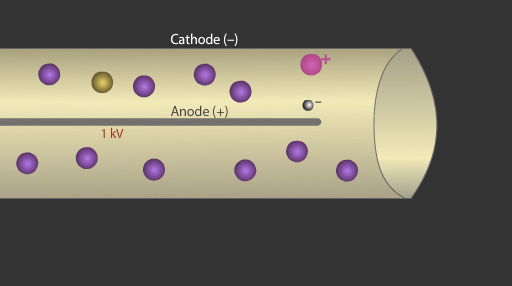
When a radioactive particle enters the tube it ionises an argon atom. The resulting electron is accelerated towards the metal wire or anode.
As the electron approaches the metal wire it experiences an increasing electric field strength which in turn applies a greater accelerating force on the electron. The accelerating force becomes so strong that on collision with other argon atoms the electron can ionise them. The electrons from these ionisations can go onto to generate a cascade of further electrons, an effect called the avalanche effect. The ionisation by one particle can result in millions of electrons striking the metal wire.
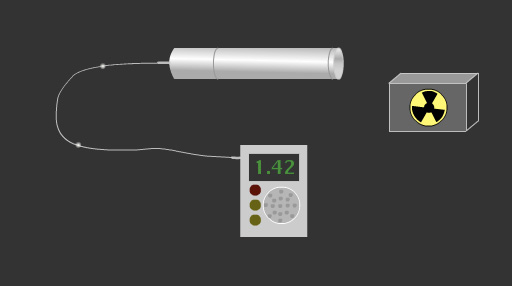
This migration of electrons inside the tube results in an electric discharge. This gives a measurable voltage pulse in the external circuit of the Geiger-Muller tube. The counter registers the number of pulses and converts them into sound signals or displays them as a measure on the screen.
Quenching Gas
The purpose of the quenching gas is to absorb the positive argon ions as they accelerate to the cathode. Without the quenching gas these positive ions will be neutralised at the cathode in an exited state or could even also dislodge electrons from the cathode. These dislodged electrons or excited atoms could trigger further ionisation creating a further voltage discharge giving inaccuracies in the measure from the device. When the quenching gas migrates to the cathode it recombines at ground state and so does not present the potential to cause any further ionisation.
