Isotopes
All the atoms of a particular element have the same number of protons. It is the number of protons which determine the chemical properties of an atom. This is because the number of protons determines the number of electrons which orbit the nucleus. The number of neutrons however can vary.
Atoms of the same element that have the same number of protons but different number of neutrons are called isotopes of that element.
Or in other words, atoms of the same element with the same atomic number but different atomic masses are called isotopes of that element.
The Three Isotopes of Hydrogen (H)
Protium 
Deuterium 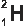
Tritium 
 Hydrogen exists as three isotopes in nature. Protium is the most common isotope. All three have the same chemical properties because they have the same proton number but have different atomic masses.
Hydrogen exists as three isotopes in nature. Protium is the most common isotope. All three have the same chemical properties because they have the same proton number but have different atomic masses.
