Alcohols
Alcohols are hydrocarbon compounds containing one or more hydroxyl group (O — H) attached to a saturated carbon atom.
Alcohol Homologous Series
The alcohols which only contain one hydroxyl group attached to a saturated carbon form a homologous series.
A hydrocarbon homologous series is a series of hydrocarbons which:
- Have the same general formula
- Differ by CH2 in molecular formulae from neighbouring compounds
- Show a gradual variation in physical properties i.e. boiling and melting point
- Have similar chemical properties
The general formula for the homologous series of alcohols is:
where n is the number of carbon atoms and OH represents the hydroxyl group.
Alcohols in the homologous series can be regarded as alkanes in which one hydrogen atom is replaced by a hydroxyl group. They are named by replacing the final “e” in the corresponding alkane by “ol”.
The table below shows the first four alcohols in the alcohol homologous series:
| Name | Number of Carbon atoms | Molecular Formula CnH2n+1OH | Structural Formula |
|---|---|---|---|
| Methanol | 1 | C1H2(1)+1OH = CH2+1OH = CH3OH | 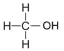 |
| Ethanol | 2 | C2H2(2)+1OH = C2H4+1OH = C2H5OH | 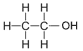 |
| Propanol | 3 | C3H2(3)+1OH = C3H6+1OH = C3H7OH | 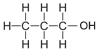 |
| Butanol | 4 | C4H2(4)+1OH = C4H8+1OH = C4H9OH | 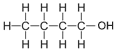 |
