Producing Ethanol by Fermentation
Aqueous solutions of ethanol can be produced when sugar solutions are fermented using yeast. The fermentation method is used to make alcoholic drinks. Fruit juices, such as grape juice, contain a source of sugar glucose (C6H12O6). When yeast is added it feeds on the sugar in the absence of oxygen to form wine (a solution of ethanol) and carbon dioxide.
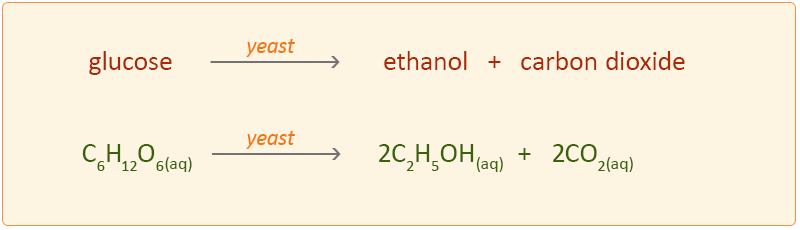
A chemical reaction called fermentation takes place in which the glucose is broken down to ethanol by the action of enzymes in the yeast.
The equation for the reaction is:
Yeast is a single cell organism and a type of fungi. It contains the enzyme zymase which acts as a catalyst for the reaction. The juices of other fruits i.e. apples, plums, pears etc. can be fermented to produce wines of various flavours.
The fermentation reaction requires the following conditions:
- Temperature – The temperature must be between the range of 25°C and 50°C. Enzymes are affected a great deal by temperature. If the temperature is too cold the enzymes move around too slowly to meet the substrate and for a reaction to occur. As the temperature increases though, so does the rate of reaction. This is because heat energy causes more collisions between the enzyme and the substrate. However, all enzymes are proteins and at too high temperatures the proteins break down. The active site of the enzyme becomes distorted and so the substrate no longer fits and hence the reaction does not occur, the enzyme is said to be denatured.
- Substrate (the glucose solution) - Enzymes work best when there is a high enough substrate concentration for the reaction they catalyse. If too little substrate is available the rate of the reaction is slowed and cannot increase any further.
- Absence of Oxygen – Air must be excluded from the vessel in which fermentation is being carried out. Air contains a large proportion of bacteria called Acetobacter. Acetobacter bacteria use atmospheric oxygen from air to oxidise ethanol in the wine, producing a weak solution of ethanoic acid (vinegar).
- Yeast – the fermentation of the glucose solution to ethanol cannot take place without the presence of yeast. Yeast contains the enzyme zymase which acts as a catalyst for the reaction
Wine contains ethanol of a concentration up to about 14 -15%. This is because above this level the ethanol kills the yeast and fermentation stops.
In countries like Brazil with limited natural oil supplies but ideal conditions for growing sugar cane, large scale fermentation is used to produce ethanol to be used as a fuel. Ethanol produced by fermentation is a renewable fuel. This is because the sugar cane can be replaced or grown again. It is also a more carbon friendly source of fuel because the glucose provided for the fermentation is produced by the plants by absorbing carbon dioxide from the atmosphere. When the ethanol is burnt, the carbon dioxide returns to the atmosphere. The long term impact of using sugar cane as a source for fuel in Brazil is that in order to meet increasing demand rainforests may be cleared to make way for the extensive land required for farming sugarcane. This will impact the local ecosystems and the sugarcane may not have the same carbon dioxide removal potential as the rainforests they replace.
