Physical Properties of Alcohols
The table below gives some of the physical properties of the first four alcohols in the alcohol homologous series:
| Name | Number of Carbon atoms | Molecular Formula CnH2n+1OH | Structural Formula | Boiling Point °C | Solubility g per 100g of water |
|---|---|---|---|---|---|
| Methanol | 1 | C1H2(1)+1OH = CH2+1OH = CH3OH | 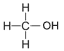 |
64 | infinite |
| Ethanol | 2 | C2H2(2)+1OH = C2H4+1OH = C2H5OH | 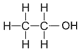 |
78 | infinite |
| Propanol | 3 | C3H2(3)+1OH = C3H6+1OH = C3H7OH | 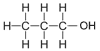 |
98 | infinite |
| Butanol | 4 | C4H2(4)+1OH = C4H8+1OH = C4H9OH | 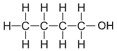 |
118 | 8.0 |
The intermolecular forces between the molecules of a compound are a determining factor in its physical properties. Intermolecular forces in compounds arise due to an imbalance of charge. In the alkanes section we discussed the effects of Van Der Waals’ intermolecular forces of attraction on the physical properties and that the strength of these forces increased with the size of the molecule.
Alcohols, like alkanes, have Van Der Waals' intermolecular attractive forces. But, they also have an additional intermolecular force of attraction due to the presence of the hydroxyl group (O—H) called hydrogen bonding.
Hydrogen Bonding in Alcohols
Hydrogen bonding is caused by the ability of particular atoms to strongly attract the electrons in a bond. A measure of how strongly an atom in a compound attracts electrons in a bond is called electronegativity. Hydrogen bonds are formed when a hydrogen atom is covalently bonded to one of the electronegative atoms, fluorine, chlorine, oxygen or nitrogen.
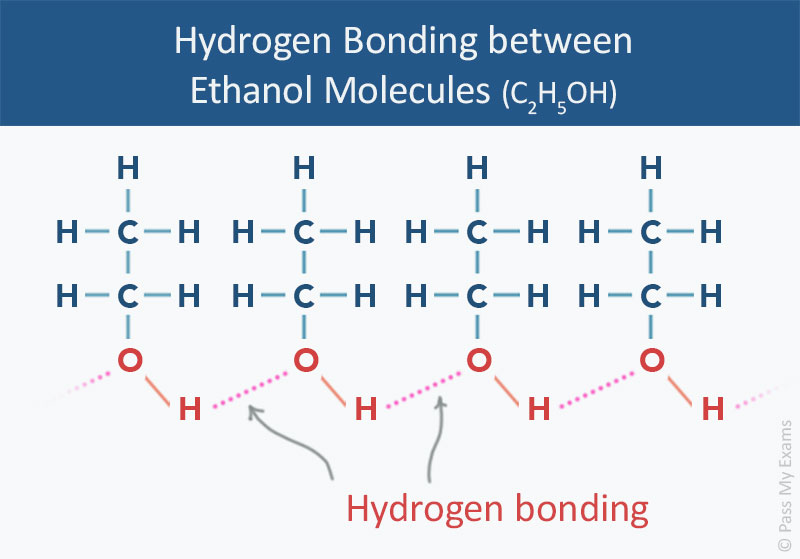
In alcohols the hydrogen bond is formed due to the covalent bonds between one oxygen atom and one hydrogen atom in the hydroxyl group (O—H). Oxygen is a highly electronegative atom and attracts the electrons in the O—H bonds towards itself. Since the proton in the nucleus of the hydrogen atom is only slightly screened the action of the oxygen pulling the electrons away from the hydrogen results in a net positive charge on the hydrogen atom. Consequently there is also a net negative charge on the oxygen atom resulting in an imbalance of charge across the hydroxyl group. The overall hydroxyl group is said to be polar because like a magnet it has two opposite charges on either ends (its poles). The net positive hydrogen atom is readily available to attract the negative electron clouds from the oxygen atom in an adjacent hydroxyl group. Unlike Van der Waals’ forces, hydrogen bonding involves a permanent imbalance of charge and therefore results in permanent dipole attractions.
The diagram below represents hydrogen bonding in ethanol molecules. Each ethanol molecule has one O—H bond and thus forms one hydrogen bond with adjacent ethanol molecules.
Boiling Point
The graph below shows a plot of the boiling points of the first four alcohols and alkanes from their respective homologous series:
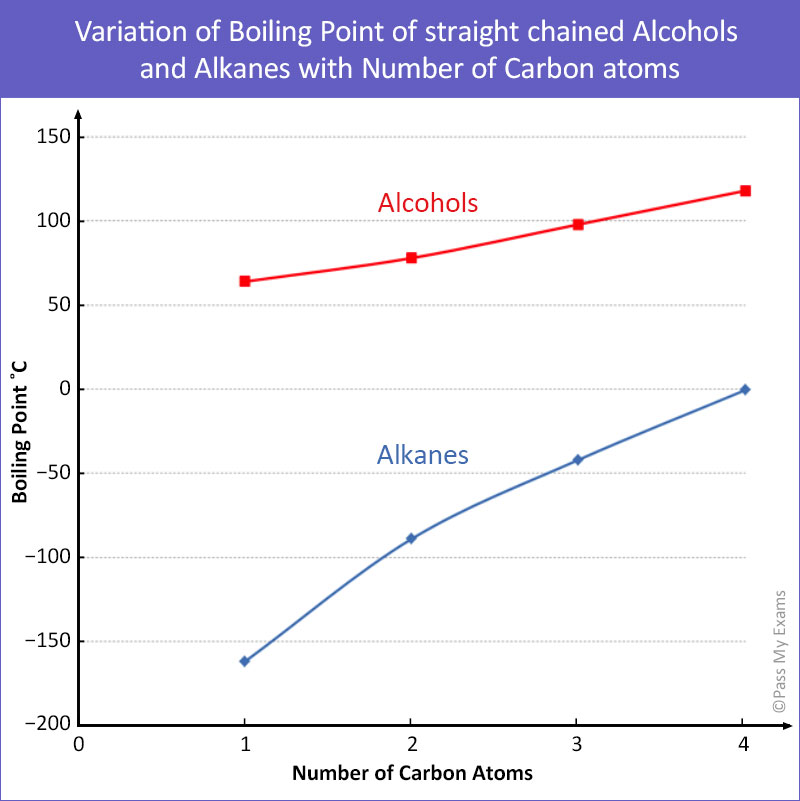
From the graph the following can be inferred:
- The boiling point of an alcohol is significantly higher than that of an alkane with the same number of carbon atoms.
- The boiling points of the alcohols increase with increasing number of carbon atoms.
The difference in the boiling points between alcohols and alkanes with the same number of carbon atoms can be explained by the following points:
- Alcohols contain the hydroxyl group (O—H) which produce intermolecular forces of attraction through hydrogen bonding. Hydrogen bonds are much stronger than Van Der Waals’ intermolecular forces. Alcohols contain two types of intermolecular forces of attraction hydrogen bonding and Van der Waals. In alkanes there are only Van Der Waal intermolecular forces of attraction. The additional forces of attraction due to hydrogen bonding between the hydroxyl groups in adjacent molecules of an alcohol make their boiling points higher than alkanes with the same number of carbon atoms.
- When comparing alcohols to alkanes it must also be remembered that for the same number of carbon atoms an alcohol is a larger molecule as one hydrogen from the alkane is replaced with a hydroxyl group. The oxygen atom in the hydroxyl group contains eight electrons and this increases the overall Van Der Waals’ intermolecular forces of attraction across the molecule. The larger Van Der Waals’ intermolecular forces experienced in an alcohol compared to an alkane of the same number of carbon atoms is also a factor in the higher boiling point.
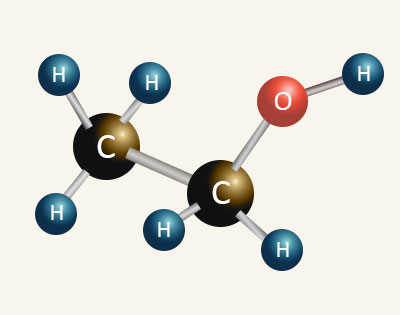
| ETHANOL Boiling Point = 78°C |
ETHANE Boiling Point = −89°C |
|---|---|
 |
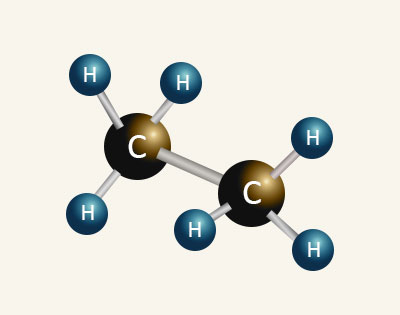 |
| When comparing ethanol to ethane we can see for the same number of carbon atoms the ethanol molecule is larger than ethane due to the inclusion of the hydroxyl (O—H) group. The hydroxyl group contains an oxygen atom which brings an extra eight electrons into the molecule which increases the Van Der Waals’ intermolecular forces of attraction between the molecules. This along with the strong forces of attractions created by hydrogen boding between the hydroxyl groups in adjacent alcohol molecules accounts for alcohols having higher boiling points than alkanes with the same number of carbon atoms. | |
The reason for the increase in boiling point with increasing carbon atoms in the alcohol homogeneous series is similar to that in the alkane homologous series. As the size and molecular mass of the molecule increases so does the number of electrons resulting in a greater imbalance in charge and hence a stronger Van der Waals’ attraction. As the alcohol molecules get larger the influence of the hydroxyl group becomes less compared to the increasingly large hydrocarbon portion. This also explains why long straight chain alcohol molecules have physical properties which tent towards the corresponding long chain alkane molecules.
Solubility of Alcohols
Solubility of alcohols falls with increasing number of carbon atoms. The small chain alcohols are good solvents and miscible with water. Methanol, ethanol and propanol are completely soluble in water in all proportions. But as the number of carbon atoms increases, solubility decreases.
| Name | Number of Carbon atoms | Molecular Formula CnH2n+1OH | Structural Formula | Solubility g per 100g of water |
|---|---|---|---|---|
| Methanol | 1 | C1H2(1)+1OH = CH2+1OH = CH3OH |  |
infinite |
| Ethanol | 2 | C2H2(2)+1OH = C2H4+1OH = C2H5OH |  |
infinite |
| Propanol | 3 | C3H2(3)+1OH = C3H6+1OH = C3H7OH |  |
infinite |
| Butanol | 4 | C4H2(4)+1OH = C4H8+1OH = C4H9OH |  |
8.0 |
| Pentanol | 5 | C5H2(5)+1OH = C5H10+1OH = C5H11OH | 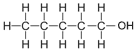 |
2.6 |
| Hexanol | 6 | C6H2(6)+1OH = C6H12+1OH = C6H13OH | 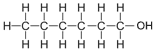 |
0.9 |
The high solubility of the small chain alcohols is due to the hydroxyl (O—H) group. The hydroxyl group is polar due to the imbalance in charge between the oxygen and hydrogen atom. This allows the hydroxyl group in the alcohol molecule to interact with polar water molecules by forming hydrogen bonds.
The animation below shows the interaction between ethanol and water molecules in order to explain the solubility of small chain alcohols:
As the number of carbon atoms in the alcohol chain gets bigger the solubility in water decreases. This is because the long chain alcohols get in between the water molecules and break the hydrogen bonds. However, the long chain molecules only have one hydroxyl group to hydrogen bond with the water molecules compared to the numerous that have been displaced. This results in many of the original hydrogen bonds in the water molecule not being replaced. Therefore, the energy required in breaking the original hydrogen bonds is not given back in the formation of new bonds and so energy considerations make long chain alcohols less soluble.
